HCOOH CH2 H2O: What It Is, Why It Matters, and Where You’ll See It
If you’ve ever looked at the formula HCOOH CH2 H2O and thought it seemed confusing, you’re not alone. But once you break it down, it’s actually a fascinating mix that shows up in chemistry labs, industrial processes, and even clean energy research. Let’s explore what this trio really means in simple terms.
Breaking Down the Formula
Think of HCOOH CH2 H2O as three puzzle pieces that work together:
- HCOOH (Formic Acid): A tiny but powerful acid, found in ants and plants, and widely used in chemical manufacturing.
- CH2 (Methylene Group): Not usually on its own, but as part of larger molecules like methanol or formaldehyde. It’s basically a carbon bridge that helps connect bigger structures.
- H2O (Water): More than just the “background solvent.” Water actively takes part in many reactions and often decides how fast or slow things happen.
Here’s a quick table to make it clearer:
| Component | What it is | Why it matters |
|---|---|---|
| HCOOH | Formic acid | Highly reactive, drives acid-base and oxidation reactions |
| CH2 | Methylene group | Acts as a connector or building block in organic chemistry |
| H2O | Water | Universal solvent, influences reaction speed and stability |
Why Chemists Care About HCOOH CH2 H2O
This isn’t just a random chemical mix—it’s a system with real value. Formic acid brings the reactivity, methylene adds structure, and water shapes the reaction environment. Together, they’re involved in processes like esterification, fuel cell development, and even the production of formaldehyde.
Everyday and Industrial Uses
You might not realize it, but this trio connects to things you see every day. Leather tanning and rubber processing use formic acid-based systems. Water plays a huge role in balancing those reactions, while methylene-based compounds show up when producing methanol and other chemicals. And in energy research, formic acid mixed with water is being tested as a cleaner fuel alternative, since it can release hydrogen in a controlled way.
Key Reactions Involving HCOOH CH2 H2O
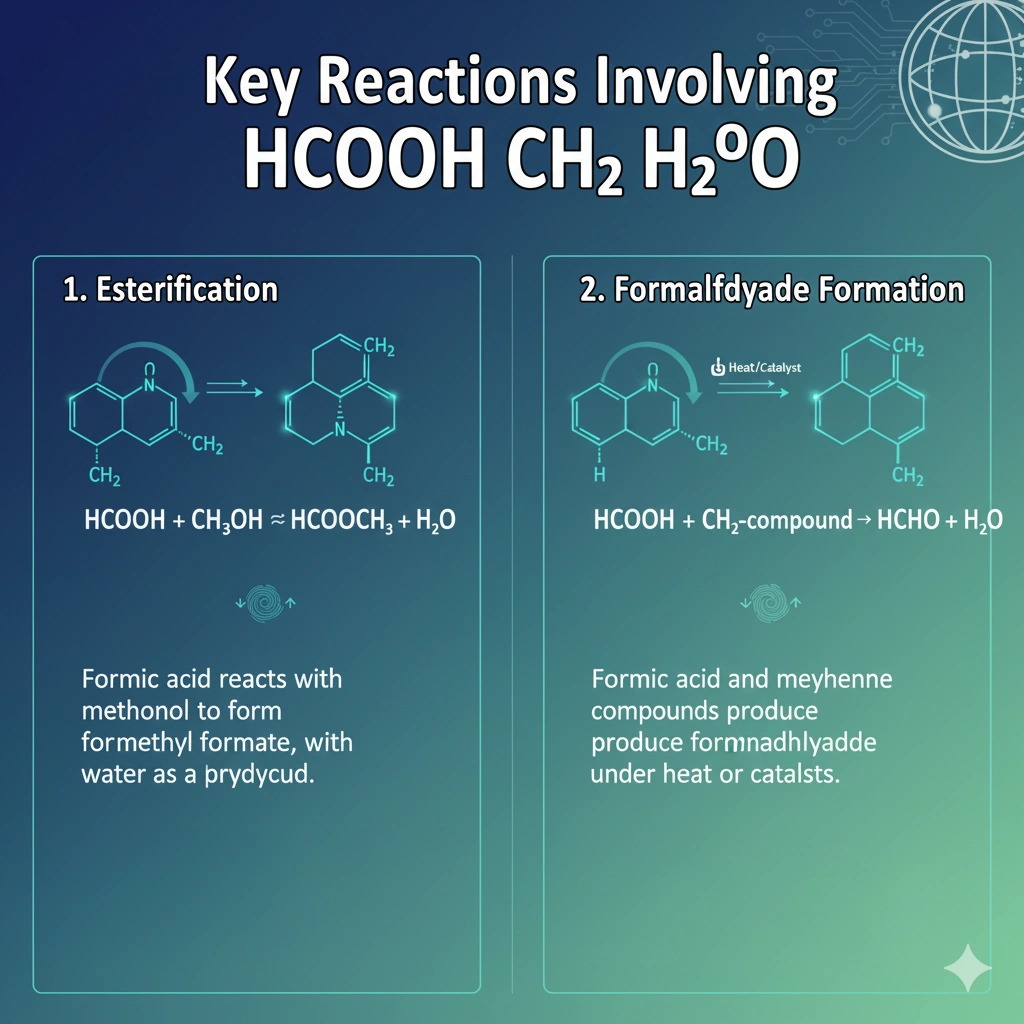
Two important reactions stand out:
- Esterification – Formic acid can react with methanol (a CH2-based compound) to form methyl formate, with water produced as a byproduct.
HCOOH + CH3OH ⇌ HCOOCH3 + H2O - Formaldehyde Formation – Under heat or catalysts, formic acid and methylene compounds may produce formaldehyde, where water again helps regulate conditions.
Safety First
As useful as this combination is, it comes with risks. Formic acid is corrosive and can cause burns or breathing problems if not handled carefully. That means gloves, goggles, and good ventilation are a must. And since some byproducts can be harmful to the environment, waste needs to be managed according to strict regulations.
What the Future Holds
Green chemistry is pushing this area forward. Imagine formic acid made from renewable biomass, or catalysts that make reactions cleaner and faster. Fuel cells powered by formic acid–water solutions are already in testing, showing promise for portable and eco-friendly energy sources.
Conclusion
So, what’s the big deal about HCOOH CH2 H2O? It’s more than just a cluster of letters and numbers—it’s a window into how chemistry powers industries, energy research, and sustainable solutions. By learning how formic acid, methylene groups, and water interact, we’re not just understanding reactions; we’re opening doors to smarter, greener ways of doing chemistry.
FAQs
Q1. What is HCOOH CH2 H2O?
HCOOH CH2 H2O is a combination of formic acid, a methylene group, and water. Together, they play a role in key chemical reactions and industrial processes.
Q2. Where is HCOOH CH2 H2O used in real life?
You’ll find it in leather tanning, rubber processing, chemical manufacturing, and even in renewable energy research like fuel cells.
Q3. Is HCOOH CH2 H2O dangerous?
Formic acid in this mix can be corrosive, so it requires proper handling with gloves, goggles, and ventilation. Safe disposal of byproducts is also essential.
Q4. How does HCOOH CH2 H2O relate to fuel cells?
Formic acid-water systems are being studied as a hydrogen source in fuel cells, offering a cleaner and more sustainable energy option.
Q5. Why is HCOOH CH2 H2O important in chemistry?
It helps explain essential reactions like esterification and formaldehyde production, making it a valuable system for both labs and industry.